TS. Đinh Hiếu Nhân
Chủ nhiệm bộ môn dược lý ĐHYD TP HCM
Giảng viên Bộ Môn Nội ĐHYD TP HCM
Phó trưởng khoa tim mạch BV Trưng Vương
Tóm tắt: Cơ sở khoa học và mục tiêu nghiên cứu: Béo phì làm nặng thêm bệnh lý tăng huyết áp và kích thích hệ thống renin – angiotensin – aldosterone (RAAS). Thuốc ức chế hệ thống RAAS thường được lựa chọn để điều trị tăng huyết áp ở bệnh nhân dư cân hay béo phì. Nghiên cứu này so sánh hiệu quả kiểm soát huyết áp và hiệu quả bảo vệ nguy cơ tim mạch của bốn thuốc ức chế hệ thống RAAS với liều tối ưu trên đối tượng bệnh nhân này.
Phương pháp: Chúng tôi tiến hành nghiên cứu phân nhóm song song, ngẫu nhiên, mù đơn, kéo dài 24 tuần trên 120 bệnh nhân dư cân hay béo phì (chỉ số khối cơ thể = BMI ≥ 27 kg/m2) có tăng huyết áp, từ 18 đến 60 tuổi. Tiêu chí chính là sự thay đổi huyết áp tâm thu và huyết áp tâm trương trung bình 24 giờ khi nhận vào nghiên cứu và khi kết thúc nghiên cứu. Huyết áp trung tâm, độ cứng động mạch, những chỉ số về chuyển hóa và tim cũng được khảo sát. Bệnh nhân được phân chia ngẫu nhiên thành các nhóm điều trị với perindopril 10mg/ ngày, enalapril 20mg/ ngày, losartan 100mg/ ngày, telmisartan 80mg/ ngày. Tất cả bệnh nhân cũng được tư vấn chế độ điều trị không dùng thuốc.
Kết quả: Giảm huyết áp tâm thu (và huyết áp tâm trương) trung bình 24 giờ có ý nghĩa thống kê (p< 0,05) khi so với mức huyết áp khi nhận vào nghiên cứu ở tất cả các nhóm: huyết áp tâm thu giảm 22 mmHg ở nhóm sử dụng perindopril, giảm 11 mmHg ở nhóm sử dụng enalapril, giảm 12 mmHg ở nhóm sử dụng losartan và giảm 15 mmHg ở nhóm sử dụng telmisartan (huyết áp tâm trương giảm tương ứng ở các nhóm lần lượt là 13 mmHg, 6 mmHg, 13 mmHg và 12 mmHg). Độ đàn hồi của động mạch chủ cải thiện với nhóm sử dụng perindopril và telmisartan. Nhóm sử dụng perindopril được quan sát thấy giảm nhiều hơn huyết áp động mạch chủ trung tâm và nồng độ leptin (30% so với 2%, 7%, và 14% ở các nhóm sử dụng enalapril, losartan và telmisartan tương ứng) (tất cả đều có ý nghĩa thống kê p < 0,05 khi so sánh với perindopril). Giảm các thông số khác ngoài huyết áp như siêu âm tim, chuyển hóa và nhân trắc học đều thấy ở các nhóm.
Kết luận: Sử dụng liều tối ưu của các thuốc trong nhóm RAAS, đặc biệt với perindopril, giúp giảm hiệu quả trị số huyết áp, cải thiện cấu trúc động mạch và điều chỉnh các yếu tố nguy cơ tim mạch ở bệnh nhân tăng huyết áp kèm dư cân hay béo phì.
1. Introduction
Effective reduction of elevated blood pressure (BP), cardiovascular prevention and mortality reduction are the main goals of antihypertensive therapy [1, 2]. Obesity not only appears to have a substantial pathophysiological effect on the haemodynamic changes seen in hypertension but also impairs the response to treatment [3–5]. Optimizing the management of hypertension in overweight and obese patients is becoming increasingly important: up to 30 % of current cases of hypertension are due to obesity, and hypertension becomes more prevalent as weight increases [6–9]. The presence of hypertension in obesity is linked to many pathological disorders, and most patients with hypertension and obesity are insulin resistant and show signs of early target-organ damage [10, 11]. Despite all of this, there are currently no international recommendations for treatment of hypertension in obese patients, principally because of a lack of randomized clinical trials in the field [2, 12]. Renin–angiotensin–aldosterone system (RAAS) inhibitors are considered the class of choice for the treatment of hypertension in obese patients because of their wide range of cardiovascular benefits [13]. Clinical evidence suggests that full-dose angiotensin-converting enzyme (ACE) inhibitor and angiotensin-receptor blocker (ARB) therapy may be useful for both correction of hypertension and prevention of cardiovascular events in hypertensive patients at high cardiovascular risk [14–16], including those with obesity [17–19]. The phase IV study described here compared four RAAS inhibitors—two ACE inhibitors (perindopril and enalapril) and two ARBs (losartan and telmisartan)—at full therapeutic doses to determine their impact on blood pressure, arterial stiffness and other cardiovascular risk factors in overweight or obese patients with hypertension.
2. Methods
This single-blind, randomized, parallel-group study included overweight or obese patients [body mass index (BMI) C27 kg/m2] with hypertension, aged 18–60 years. Eligible patients were untreated or were hypertensive after a 2-week washout period, with clinic brachial systolic blood pressure (SBP) C140 to\160 mmHg in a sitting position and/or diastolic blood pressure (DBP) C90 to\100 mmHg (measured by the Korotkov method, i.e. three measurements at intervals of 1–2 min; BP was the mean of the last two measurements). A special cuff was used for measuring BP in obese patients. Patients were excluded if they were aged\18 years or had known hypersensitivity, intolerance or contraindications to ACE inhibitors or ARBs; unstable angina; heart failure; renal or hepatic insufficiency; grade 2 or 3 arterial hypertension (C160/100 mmHg); a history of stroke; diagnosed or suspected secondary hypertension; or a serious illness affecting their prognosis. Stable coronary artery disease was managed solely with beta-blocker therapy.
The primary endpoint of the study was the change in mean 24-h SBP and DBP from baseline to study end.
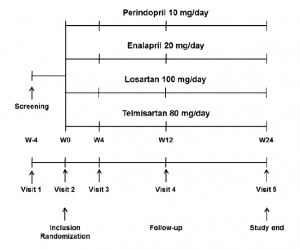
Fig. 1 Trial design: comparison of perindopril, enalapril, losartan and telmisartan in overweight patients with hypertension. W week
Secondary endpoints were the change in central aortic BP and arterial elasticity. Other parameters investigated included echocardiographic and metabolic indices.
Using an envelope method, patients were randomly allocated to four groups: perindopril 10 mg/day (Servier, Suresnes, France); enalapril 20 mg/day (Merck Sharp & Dohme, Whitehouse Station, NJ, USA); losartan 100 mg/ day (Merck Sharp & Dohme); or telmisartan 80 mg/day (Boehringer Ingelheim, Ingelheim, Germany). Patients were blinded to their treatment allocation and took the treatment for 24 weeks in addition to implementing recommended nonpharmacological interventions. These interventions included lifestyle modification and weight loss (diet, physical activity) and were initiated 3 months before treatment allocation. Weight loss drugs were not allowed. All of the patients in the study gave informed consent before inclusion, and the protocol was approved by the regional ethical committee of the Volgograd State Medical University. The study design is shown in Fig. 1.
Clinical and demographic characteristics of the groups were assessed at baseline, while parameters of BP, echocardiography, vascular structure, blood biochemistry and anthropometry were assessed at baseline and 24 weeks. Treatment tolerability was assessed at each follow-up visit. Ambulatory blood pressure monitoring (ABPM) [using a Spacelabs 90207 machine; Spacelabs Medical Inc., Issaquah, WA, USA] was used to determine mean 24-h daytime and night-time SBP and DBP, with measurements every 15 min during the day (0700–2300 hours) and every 30 min during the night (2300–0700 hours). A minimum of 60 BP measurements were required for ABPM data to be considered valid for analysis.
An Aloka Prosound L7 Premier device (Hitachi Aloka Medical Ltd., Tokyo, Japan) was used to determine echocardiographic parameters (ejection fraction, stroke index, end-systolic and end-diastolic diameters, and intima/media thickness). Pulse wave velocity (PWV), a parameter of vascular stiffness, was studied using a computerized Complior device (Colson, Garges-les-Gonesses, France), as described elsewhere [20]. The augmentation index and central aortic BP were determined using a SphygmoCor device (AtCor Medical Pty Ltd., West Ryde, NSW, Australia).
Fasting blood samples were collected for biochemistry [lipids, glucose, glycosylated haemoglobin, C-peptide, leptin, immunoreactive insulin (IRI), uric acid and creatinine levels]. Serum leptin was determined using the standard DSL enzyme immunoassay set (Diagnostics System Laboratories Inc., Webster, TX, USA), and C-peptide levels were measured using a C-peptide immunoluminometric assay. Impaired glucose tolerance (IGT) and diabetes mellitus were determined by fasting and 2-h measurements of plasma glucose levels following oral administration of 75 g glucose. IGT was established by a fasting glucose level Fig. 1 Trial design: comparison of perindopril, enalapril, losartan and telmisartan in overweight patients with hypertension. W week \7.0 mmol/L and a 2-h glucose level C7.8 mmol/L, while diabetes mellitus was established by a fasting glucose level C7.0 mmol/L and a 2-h glucose level C11.1 mmol/L. Percentage body fat was determined using an Omron BF306 device (OMRON Healthcare Inc., Lake Forest, IL, USA).
Data are presented as means ± standard deviations or numbers and percentages. A paired student’s t test was used to detect significant changes with treatment; p\0.05 was considered statistically significant. Continuous quantitative baseline and demographic features were tested using a simple t test on independent samples. A nonstandard distribution of values was analyzed using a Mann–Whitney test, while qualitative traits were assessed using a Fisher’s exact test or v2 test, depending on the number of observations in each cell of the contingency table. Comparison of the intergroup effectiveness of various treatments was performed using Dunnett’s test, which assessed changes in the rate of improvement, compared with baseline, and standardized them (a = 5 %). Differences between measurements obtained after active treatment and the corresponding baseline values were expressed as means ± standard deviations and were compared through analysis of variance (ANOVA), with allowance for the treatment order and subject. If the p value associated with the main factor (treatment) fell below 0.05, single contrasts between the four treatments were tested with the Newman–Keuls test. p values \0.05 were considered statistically significant. Statistical processing was performed using BMDP statistics software (Statistical Solutions, Saugus, MA, USA).
3. Results
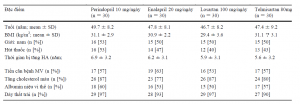
The baseline characteristics of the 120 patients (61 men, 59 women) are shown in Table 1. The mean age of the population was 48 years, the mean BMI was 30.6 kg/m2, and the mean duration of hypertension was 6.2 years. Most patients had left ventricular hypertrophy (93 %) and hypercholesterolaemia (83 %); over half had microalbuminuria (57 %) and a history of coronary artery disease (57 %); and half were smokers (47 %). There were 30 patients in each treatment group, and there were no statistically significant between-group differences at baseline. There were no dropouts during the study, and no safety issues were recorded. No changes in beta-blocker dosage in patients with stable coronary artery disease were reported. .
3.1. Changes in 24-h Blood Pressure
The absolute reductions in mean 24-h SBP at 24 weeks versus baseline for perindopril, enalapril, losartan and telmisartan (all p\0.05) were -22, -11, -12 and -15 mmHg, respectively, which represents reductions of 14, 7, 8, and 10 % (Table 2; Fig. 2). Absolute reductions in mean 24-h DBP at 24 weeks versus baseline for perindopril, enalapril, losartan and telmisartan (all p\0.05) were -13, -6, -13 and -12 mmHg, respectively, which represents reductions of 13, 6, 13 and 12 % (Table 2). The reductions in 24-h SBP and DBP were most pronounced with perindopril [p\0.05 versus other treatments] (Fig. 2), as were the reductions in clinic brachial SBP and DBP (p\0.05 versus baseline). All treatments significantly reduced daytime and night-time BP versus baseline, but the magnitude of the reduction was greater with perindopril (p\0.05) than with the other agents (Table 2). After 24 weeks, all of the RAAS inhibitors had improved BP dipping profiles. Perindopril, losartan and telmisartan restored the normal dipping profile in 85 % of patients, while enalapril restored the normal dipping profile in 65 % of patients. Perindopril and telmisartan had positive effects on 24-h BP profile normalization, with significant reductions in early morning surges (EMS) in SBP (p\0.05 versus baseline); there was no significant reduction in EMS with enalapril or losartan.
3.2. Changes in Vascular Structure and Central Blood Pressure
Vascular structure parameters improved significantly with perindopril and telmisartan by 24 weeks (Table 3). Carotid– femoral PWV fell by 29 and 24 % with perindopril and telmisartan, respectively (p\0.05 versus baseline), and carotid–radial PWV fell significantly by 26 % (p\0.05) with perindopril. Perindopril had the most pronounced effect on the thickness of the intima/media complex. The perindopril-associated reduction in the augmentation index was 18 % (p\0.05 versus baseline) versus nonsignificant reductions of 2 % with enalapril, 5 % with losartan and 13 % with telmisartan. The corresponding reductions in central aortic pressure were 8 % (p\0.05 versus baseline), and\1, 1 and 4 %, respectively [all nonsignificant (p = NS)].
3.3. Cardiac Remodelling and Diastolic Dysfunction
After 24 weeks, all four drugs reduced posterior left ventricular wall thickness (by 5,\1,\1 and\1 % for perindopril, enalapril, losartan and telmisartan, respectively) and left ventricular mass index (by 14, 3, 6, and 10 %, respectively) (Table 4). These reductions were significant only with perindopril (p\0.05 versus baseline). Treatment with perindopril also led to a significant improvement in the early/atrial (late) ventricular filling velocity (E/A) ratio, a marker of diastolic dysfunction. There were no significant changes in other echocardiographic parameters with any of the treatments.
Baseline: Thời điểm nhận vào nghiên cứu
* P<0,05 so với tỉ lệ cải thiện ( so thời điểm nhận vào nghiên cứu với sau 24 tuần) của nhóm điều trị bằng perindopril, ϯ p< 0.05 so với perindopril
3.4. Metabolic and Anthropometric Parameters
There were significant between-group differences with regard to leptin, C-peptide, and lipid and carbohydrate metabolism at 24 weeks (Table 5). Leptin was reduced by 30 % with perindopril (p\0.05 versus baseline) versus 2, 7 and 14 % with enalapril, losartan, and telmisartan, respectively (all p\0.05 versus perindopril). C-peptide was significantly lower at 24 weeks in the perindopril and telmisartan groups. Treatment with perindopril and telmisartan led to improvement of the lipid profile, while the effect of enalapril and losartan was neutral. After 24 weeks, all therapies had a modest favourable effect on glucose metabolism.
Over the course of the study, there were no increases in BMI or the waist/hip circumference ratio with any of the treatments (Table 5). There were trends toward a reduction in BMI with enalapril, losartan and telmisartan and a significant reduction in BMI with perindopril (p\0.05 versus baseline). The percentage body fat was also reduced significantly with perindopril (p\0.05). The waist/hip circumference ratio was reduced significantly with perindopril and telmisartan (p\0.05 versus baseline).
4. Discussion
To our knowledge, ours is the first study to compare the antihypertensive, nephroprotective, cardioprotective and metabolic effects of two ACE inhibitors (perindopril and enalapril) and two ARBs (losartan and telmisartan) administered at full dose in overweight or obese patients with hypertension. We found large reductions in blood pressure, improved arterial elasticity and regulation of a wide range of cardiovascular disease risk factors with RAAS inhibition in these patients. RAAS inhibitors at full dose appear to be a viable therapeutic strategy for hypertension in obesity, in terms of etiological, pathogenetic and symptomatic objectives. RAAS inhibitors also protect target organs, reduce the risk of diabetes and positively influence metabolism [21].
Patients in our study were initiated on antihypertensive monotherapy at full dose, according to European guideline recommendations, even though most obese patients with hypertension are ultimately likely to require multiple antihypertensive drugs to control BP [2, 13]. All RAAS inhibitors, by definition, reduce hypertension by blocking the RAAS, but the way in which ACE inhibitors and ARBs achieve this differs. ARBs block angiotensin 1 receptors, preventing their activation by angiotensin II, a powerful vasoconstrictor and mediator of inflammation, lipid accumulation and thrombogenesis. In contrast, ACE inhibitors impair the production of angiotensin II by inhibiting the enzyme ACE, which converts angiotensin I to angiotensin II [22]. By inhibiting this enzyme, ACE inhibitors also prevent degradation of the vasodilatory and cardioprotective peptide bradykinin [23]. Bradykinin stimulates the release of other important vasodilators like nitric oxide, prostacyclin and endothelium-derived hyperpolarizing factor, providing cardioprotective benefits and endothelial protection against remodelling, atherosclerosis and thrombosis [24]. Preservation of bradykinin together with reduced angiotensin II production with ACE inhibitors may explain some of the clinical differences between ACE inhibitors and ARBs in terms of cardiovascular outcomes [25].
Predominantly hydrophilic ACE inhibitors, like enalapril and lisinopril, may be poorly distributed in adipose tissue, and this may affect the production of adipokines. Secretion of adipokines is closely linked to the activity of RAAS components, primarily angiotensin II [18, 26, 27]. Lipophilic ACE inhibitors, like perindopril and ramipril, positively inhibit the production of adipokines at the cellular level, which is considered beneficial [18, 26]. Liposolubility and tissue ACE selectivity are related, and perindopril is known to have one of the highest tissue ACE selectivities of all ACE inhibitors [28]. Increased liposolubility of ACE inhibitors also means better penetration of atherosclerotic plaque, leading to a greater antiatherosclerotic effect [29].
Perindopril’s capacity to protect endothelium against the deleterious effects of atherosclerosis and its long duration of antihypertensive effect (trough/peak ratio 75–100 %) distinguish it from other ACE inhibitors [14, 30–32]. Perindopril is known to be quickly and extensively absorbed, and is an effective RAAS inhibitor that can elicit a response in patients unresponsive to therapy with other ACE inhibitors or ARBs [33, 34]. In our study, 24-h SBP and DBP, as well as daytime and night-time BP, fell more with perindopril than with enalapril, losartan or telmisartan. Moreover, the importance of the long duration of action was underlined by a reduction with perindopril and telmisartan of early morning BP surges, which are known to predict cardiovascular events [35].
Patients treated with perindopril also displayed a significant reduction in carotid intima/media thickness, a marker of vascular remodelling. This reduction indicates that perindopril is able to preserve blood vessel structure. Along with its proven ability to reduce blood pressure, this may explain why treatment with perindopril led to a statistically significant improvement in PWV, a marker of arterial stiffness. Arterial stiffness is strongly correlated with cardiovascular events and all-cause mortality [36].
ACE inhibitors are known to have a positive effect on several conditions associated with hypertension and obesity, such as left ventricular hypertrophy, congestive heart failure, renal hyperfiltration and microalbuminuria, and to be associated with reductions in bodyweight [19]. The moderate degree of obesity in our population could explain the improvement in anthropometric parameters and the decrease in body fat in our study, particularly with perindopril. Even though BMI in the perindopril group fell by 2 points, the impact of BMI reduction on BP reduction could not be determined with certainty.
The efficacy of RAAS inhibition with perindopril in overweight and obese patients with hypertension has been shown previously, and perindopril is also known for its cardioprotective, angioprotective and nephroprotective effects [37–42]. Earlier studies have also demonstrated the benefits of full-dose perindopril therapy in lowering blood pressure and protecting blood vessels in hypertension, as well as in modulating leptin levels [15, 43–45]. Metabolic dysfunction (hyperlipidaemia, hyperglycaemia and hyperleptinaemia) in overweight or obese patients with hypertension also appears to be corrected by perindopril.
It is important that antihypertensive agents used by overweight or obese patients modulate adipokine levels because of adverse cardiometabolic effects [46]. The significant reduction in leptin levels with perindopril in our study may be related, at least in part, to the reduction in BMI in the perindopril group. Our results do, however, confirm previous findings that show this effect is not class specific, e.g. enalapril does not have this effect [27]. Perindopril may also positively modulate resistin and adiponectin [18, 26].
Positive effects of full-dose RAAS inhibitors on serum lipid profiles also appear not to be class specific. The efficacy of perindopril in overweight patients has already been confirmed in other studies, including a Russian study in which 70 % of patients were overweight and a French study in which a third of patients were obese [37, 47]. Improvement of blood lipids, particularly triglycerides, should not be considered a random finding. A study in patients with hypertension and type 2 diabetes showed that perindopril increased high-density–lipoprotein cholesterol by 0.16 mmol/L (p\0.05) after 1 year, while a trial in hypertensive patients showed that it reduced triglycerides levels by 0.6 mmol/L (p\0.05) [48, 49].
Treatment with ACE inhibitors in general—and perindopril in particular—is associated with restoration of the early peak of insulin secretion, improvement in carbohydrate metabolism and a reduction in insulin resistance [37, 50]. Insulin resistance can lead to severe endothelial dysfunction, increased vascular tone and development of proliferative processes in the vessel wall. ACE inhibitors reduce angiotensin II, a competitive antagonist of insulin that reduces peripheral glucose uptake by cells, which accelerates glucose oxidation and decreases endogenous glucose production. As regards the effect of perindopril on metabolism, further investigation is required, as study results have been mixed. One study in overweight patients with essential hypertension found that perindopril had a neutral effect on metabolism, while a comparative study versus losartan showed that perindopril was useful for improving insulin sensitivity and decreasing insulin resistance in obese patients [51, 52].
Our study had the typical limitations of a single-blind phase IV study. The absence of double-blinding and randomization meant that an equal balance between the four groups in terms of age, baseline SBP and BMI could not be ensured and may have impacted the results of the study. The small size of the population limited the conclusions that could be drawn. The mean BMI of the population indicated borderline obesity, though this did not preclude observations of effects on obesity-related parameters. Ongoing nonpharmacological interventions may have affected the results, but these interventions occurred equally in all groups.
5. Conclusion
Effective full-dose RAAS inhibition appears to be an important therapeutic option in overweight or obese patients with hypertension. Full-dose perindopril may be the most suitable RAAS inhibitor for overweight or obese patients with hypertension, since not only does it effectively reduce BP, but it also appears to regulate a wide range of cardiovascular risk factors associated with hypertension and obesity.
Acknowledgments
No funding was provided for this study. S. Nedogoda has received honoraria from several companies,
including Servier, Takeda, KRKA, Abbott, Novartis, Boehringer Ingelheim and Astra Zeneca. The other authors have no conflicts of interest to declare. The authors have no other relevant affiliations or financial involvement with any organization or entity in conflict with the subject matter or materials discussed in the manuscript, apart from those disclosed.
References
1. Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007;28(12):1462–536.
2. Mancia G, Laurent S, Agabiti-Rosei E, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens. 2009;27(11):2121–58.
3. Schmieder RE, Gatzka C, Schachinger H, et al. Obesity as a determinant for response to antihypertensive treatment. BMJ. 1993;307(6903):537–40.
4. Jordan J, Engeli S. Obesity, hypertension, and cardiovascular health: is there anything poor Cassandra tries to tell us? J Hypertens. 2012;30(6):1103–5.
5. Schmieder RE, Rockstroh JK. Obesity and hypertension. Curr Opin Nephrol Hypertens. 1994;3(5):546–9.
6. Gortmaker SL, Swinburn BA, Levy D, et al. Changing the future of obesity: science, policy, and action. Lancet. 2011;378(9793): 838–47.
7. Ludwig DS. Childhood obesity—the shape of things to come. N Engl J Med. 2007;357(23):2325–7.
8. MacMahon SW, Blacket RB, Macdonald GJ, et al. Obesity, alcohol consumption and blood pressure in Australian men and women. The National Heart Foundation of Australia Risk Factor Prevalence Study. J Hypertens. 1984;2(1):85–91.
9. Bramlage P, Pittrow D, Wittchen HU, et al. Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. Am J Hypertens. 2004;17(10):904–10.
10. Dorresteijn JA, Visseren FL, Spiering W. Mechanisms linking obesity to hypertension. Obes Rev. 2012;13(1):17–26.
11. Kurukulasuriya LR, Stas S, Lastra G, et al. Hypertension in obesity. Med Clin North Am. 2011;95(5):903–17.
12. Whitworth JA. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21(11):1983–92.
13. Jordan J, Yumuk V, Schlaich M, et al. Joint statement of the European Association for the Study of Obesity and the European Society of Hypertension: obesity and difficult to treat arterial hypertension. J Hypertens. 2012;30(6):1047–55.
14. Fox KM. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet. 2003;362(9386):782–8.
15. Tropeano AI, Boutouyrie P, Pannier B, et al. Brachial pressureindependent reduction in carotid stiffness after long-term angiotensin- converting enzyme inhibition in diabetic hypertensives. Hypertension. 2006;48(1):80–6.
16. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensinconverting- enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342(3):145–53.
17. Kalil GZ, Haynes WG. Sympathetic nervous system in obesityrelated hypertension: mechanisms and clinical implications. Hypertens Res. 2012;35(1):4–16.
18. Nakamura T, Kawachi K, Saito Y, et al. Effects of ARB or ACEinhibitor administration on plasma levels of aldosterone and adiponectin in hypertension. Int Heart J. 2009;50(4):501–12.
19. Sharma AM, Pischon T, Engeli S, et al. Choice of drug treatment for obesity-related hypertension: where is the evidence? J Hypertens. 2001;19(4):667–74.
20. Asmar R, Topouchian J, Pannier B, et al. Pulse wave velocity as endpoint in large-scale intervention trial. The Complior study. Scientific, Quality Control, Coordination and Investigation Committees of the Complior Study. J Hypertens. 2001;19(4):813–8.
21. Manrique C, Lastra G, Gardner M, et al. The renin angiotensin aldosterone system in hypertension: roles of insulin resistance and oxidative stress. Med Clin North Am. 2009;93(3):569–82.
22. Dzau VJ, Bernstein K, Celermajer D, et al. The relevance of tissue angiotensin-converting enzyme: manifestations in mechanistic and endpoint data. Am J Cardiol. 2001;88(9 suppl):1L–20L.
23. Ceconi C, Francolini G, Olivares A, et al. Angiotensin-converting enzyme (ACE) inhibitors have different selectivity for bradykinin binding sites of human somatic ACE. Eur J Pharmacol. 2007; 577:1–6.
24. Morishita T, Tsutsui M, Shimokawa H, et al. Long-term treatment with perindopril ameliorates dobutamine-induced myocardial ischemia in patients with coronary artery disease. Jpn J Pharmacol. 2002;88(1):100–7.
25. van Vark LC, Bertrand M, Akkerhuis KM, et al. Angiotensinconverting enzyme (ACE) inhibitors reduce mortality in hypertension—a meta-analysis of randomized clinical trials of renin-angiotensin-aldosterone-system (RAAS) inhibitors involving 158,998 patients. Eur Heart J. 2012;33(16):2088–97.
26. Krysiak R, Sierant M, Marek B, et al. The effect of perindopril and enalapril on plasma resistin levels in normotensive patients with coronary heart disease. Endokrynol Pol. 2010;61(6):683–90.
27. Krysiak R, Sierant M, Marek B, et al. The effect of angiotensinconverting enzyme inhibitors on plasma adipokine levels in normotensive patients with coronary artery disease. Endokrynol Pol. 2010;61(3):280–7.
28. Ferrari R. Angiotensin-converting enzyme inhibition in cardiovascular disease: evidence with perindopril. Expert Rev Cardiovasc Ther. 2005;3(1):15–29.
29. Ferrari R, Fox K. Insight into the mode of action of ACE inhibition in coronary artery disease: the ultimate ‘EUROPA’ story. Drugs. 2009;69(3):265–77.
30. Fox KM. Management of coronary artery disease: implications of the EUROPA trial. Br J Cardiol. 2004;11(3):195–204.
31. Physicians’ desk reference. 58th ed. Montvale: PDR Network LLC, 2004.
32. Morgan T, Anderson A. Duration of antihypertensive effect of perindopril (P), enalapril (E) and captopril (C). Hypertension. 1993;21(4):568.
33. Devissaguet JP, Ammoury N, Devissaguet M, et al. Pharmacokinetics of perindopril and its metabolites in healthy volunteers. Fundam Clin Pharmacol. 1990;4(2):175–89.
34. Guo W, Turlapaty P, Shen Y, et al. Clinical experience with perindopril in patients nonresponsive to previous antihypertensive therapy: a large US community trial. Am J Ther. 2004;11(3): 199–205.
35. Stolarz-Skrzypek K, Thijs L, Richart T, et al. Blood pressure variability in relation to outcome in the International Database of Ambulatory Blood Pressure in Relation to Cardiovascular Outcome. Hypertens Res. 2010;33(8):757–66.
36. Blacher J, Asmar R, Djane S, et al. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33(5):1111–7.
37. Nedogoda S. Efficiency of perindopril in patients with arterial hypertension and obesity [in Russian]. Kardiologiia. 2011;51(11): 38–44.
38. Cordonnier DJ, Pinel N, Barro C, et al. Expansion of cortical interstitium is limited by converting enzyme inhibition in type 2 diabetic patients with glomerulosclerosis. The Diabiopsies Group. J Am Soc Nephrol. 1999;10(6):1253–63.
39. Hermans MP, Brichard SM, Colin I, et al. Long-term reduction of microalbuminuria after 3 years of angiotensin-converting enzyme inhibition by perindopril in hypertensive insulin-treated diabetic patients. Am J Med. 1992;92(4B):102S–7S.
40. Rodriguez-Granillo GA, Vos J, Bruining N, et al. Long-term effect of perindopril on coronary atherosclerosis progression (from the PERindopril’s Prospective Effect on Coronary aTherosclerosis by Angiography and IntraVascular Ultrasound Evaluation [PERSPECTIVE] Study). Am J Cardiol. 2007;100(2): 159–63.
41. Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366(9489): 895–906.
42. Ceconi C, Fox KM, Remme WJ, et al. ACE inhibition with perindopril and endothelial dysfunction. Results of a substudy of the EUROPA study: PERTINENT. Cardiovasc Res. 2007;73: 237–46.
43. Myers MG. A dose-response study of perindopril in hypertension: effects on blood pressure 6 and 24 h after dosing. Perindopril Multicentre Dose-Response Study Group. Can J Cardiol. 1996; 12(11):1191–6.
44. Tsoukas G, Anand S, Yang K. Dose-dependent antihypertensive efficacy and tolerability of perindopril in a large, observational, 12-week, general practice-based study. Am J Cardiovasc Drugs. 2011;11(1):45–55.
45. Ficek J, Kokot F, Chudek J, et al. Influence of antihypertensive treatment with perindopril, pindolol or felodipinon plasma leptin concentration in patients with essential hypertension. Horm Metab Res. 2002;34(11–12):703–8.
46. Harwood HJ Jr. The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology. 2012;63:57–75.
47. Poggi L, Renucci JF, Denolle T. Treatment of essential hypertension in general practice: an open-label study of 47,351 French hypertensive patients treated for one year with perindopril. Can J Cardiol. 1994;10(Suppl):D21D–4D.
48. Jandrain B, Herbaut C, Depoorter JC, et al. Long-term (1 year) acceptability of perindopril in type II diabetic patients with hypertension. Am J Med. 1992;92(4B):91S–4S.
49. Andrejak M, Santoni JP, Carre A, et al. A double-blind comparison of perindopril and hydrochlorothiazide-amiloride in mild to moderate essential hypertension. Fundam Clin Pharmacol. 1991;5(3):185–92.
50. Marre M, Leye A. Effects of perindopril in hypertensive patients with or without type 2 diabetes mellitus, and with altered insulin sensitivity. Diabetes Vasc Dis Res. 2007;4(3):163–73.
51. Bohlen L, Bienz R, Doser M, et al. Metabolic neutrality of perindopril: focus on insulin sensitivity in overweight patients with essential hypertension. J Cardiovasc Pharmacol. 1996;27(6): 770–6.
52. Fogari R, Mugellini A, Zoppi A, et al. Losartan and perindopril effects on plasma plasminogen activator inhibitor-1 and fibrinogen in hypertensive type 2 diabetic patients. Am J Hypertens. 2002;15(4):316–20.
SERV-HTN-05-07-2022-2





Xin vui lòng đăng nhập để nhận xét vào bài viết này.